CALES researchers drive promising C. diff treatments through development
Gayatri Vedantam, V.K. Viswanathan and their research team are conducting pre-clinical tests on two potential treatments for a disease that affects nearly half a million Americans each year.
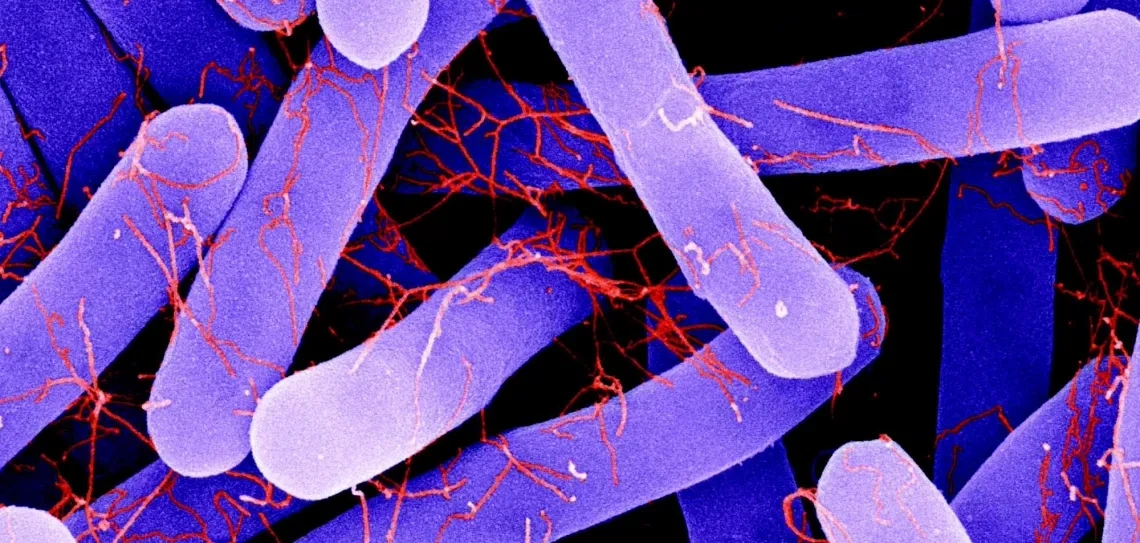
It’s the bane of hospitals around the world: a healthcare-associated infection caused by a bacterium called Clostridiodes difficile (C. diff). Infection with this intestinal pathogen causes severe diarrheal disease in both humans and animals and can be fatal in some cases. The Centers for Disease Control and Prevention (CDC) has designated C. diff an “Urgent Threat” to healthcare in the U.S., estimating that it infects nearly half a million Americans each year.
Researchers in the School of Animal and Comparative Biomedical Sciences (ACBS) at the University of Arizona aim to change that.

Vedantam, Viswanathan and the Collaboratory for Anti-infective and Therapeutic Strategies (CATS).
Under the leadership of ACBS professors Gayatri Vedantam and V.K. Viswanathan and their Collaboratory for Anti-Infectives and Therapeutic Strategies (CATS), are developing novel treatment strategies to protect against C. diff and other gastrointestinal pathogens. Two promising treatments – a preventative called Syn-LAB and a therapeutic called SRPNT – have recently been granted patents and are undergoing extensive pre-clinical testing to ensure safety and efficacy in advance of clinical trials.
Pre-clinical testing, de-risking and the “Valley of Death”
Pre-clinical testing is an essential phase in the development of any novel medical treatment. Vedantam describes the process as one of “de-risking” – that is, identifying and mitigating all possible risks associated with a new therapeutic before moving on to clinical trials.
“It involves two arms,” she explained. “The first is in vitro validation and testing – essentially, testing in test tubes to ensure that the drug can be made without variation between batches and that it works the way we expect it to. The second is in vivo efficacy – ensuring that the drug works like it’s supposed to in animal models of infection.”
According to Vedantam, both Syn-LAB and SRPNT have passed in vitro and in vivo tests with flying colors. Now, they’re moving onto larger field testing as well as pharmacology (safety and dosing) assessments.
“Once we do all this de-risking, we can be very comfortable moving on to clinical trials,” she said. “We know how the products work. We know how much to give, how long to give it, how long it stays in the body. And we know if there are any side effects.”
It’s not unusual for the pre-clinical testing phase to take several years, and not all products make it through the process. Many promising therapeutics disappear into what many in the scientific community call the “Valley of Death.”
“A lot of drugs or products that do very well in the early stages of pre-clinical testing never see the light of day,” said Vedantam. “If you don’t go above and beyond to ensure safety and efficacy, you’ll never make it to a clinical trial. That’s why we’re approaching this phase very deliberately and making sure we’re pulling out every tool in the toolbox to make sure our products are successful.”
Vedantam credits a network of collaborators in and out of the lab with getting Syn-LAB and SRPNT to this point. “We have a phenomenal team of undergraduate and graduate students, postdocs and senior scientists working on these projects, to say nothing of our partners in the medical and veterinary community” she said. “And we’ve received outstanding support from CALES, the university and Tech Launch Arizona throughout the process.”
One disease, two approaches
CATS has developed both Syn-LAB and SRPNT to protect against C. diff infection, but from different directions. Syn-LAB aims to prevent infection; SRPNT hopes to cure any infections that do occur.
Vedantam explained that in U.S. hospitals, patients frequently get broad-spectrum antibiotics, either as a preventative against potential infection following a procedure or as a treatment for active infections. Unfortunately, those types of antibiotics kill both the “bad,” infectious bacteria as well as the “good” bacteria that normally populate the gut. This opens the door for C. diff to enter and colonize the GI tract, causing intestinal damage and diarrhea.
“When gut microbiota are disrupted, C. diff can get into the host and colonize those niches in the gut that are now vacant,” she said. “There’s no vaccine to prevent it, and the only treatment is more antibiotics. So the good bacteria in the GI tract just never come back to baseline, and C. diff can just sit there and keep causing cycles of infection.”
Vedantam and CATS have developed Syn-LAB, a synthetic lactobacillus bacterium, to prevent C. diff. infections by occupying those vacant niches in the gut before the pathogen can take hold. It colonizes the GI tract in the same way that C. diff does so that the organism can’t establish itself there. Once the risk of C. diff infection has passed, Syn-LAB self-destructs, allowing the good microbiota to return to the gut.
On the treatment side of the problem, CATS has developed a resistance-defying, precision anti-infective called SRPNT (or “serpent,” as it’s affectionately called by the research team).
“These drugs are based on a platform into which we can add or subtract little blocks of RNA specific to the pathogen we’re trying to target,” Vedantam explained. “SRPNT is a treatment designed exclusively to knock out C. diff – and only C. diff. It leaves all the other millions of bacteria in the GI tract alone.”
Vedantam and her collaborators believe these treatments have the potential to protect millions of people and animals from C. diff infection. They’re currently completing final pre-clinical studies and expect to be ready for clinical trials in humans in the next two years.
“We have a lot of work to do,” she said. “But we have an enormously talented team committed to this work and this process. I cannot say enough about how proud we are to be able to work with them.”
