Naranjo, S.E., L. Cañas, and P.C. Ellsworth. 2/2004. Mortalidad de Bemisia tabaci en un sistema de cultivos múltiples. Horticultura Internacional. pp. 14-21.
Figure 1. The sweetpotato
whitefly, Bemisia tabaci, is a cosmopolitan pest of many field and horticultural
crops where is causes direct feeding damage, transmits viruses, cause
plant physiological disorders, and contaminates harvested products through
the excretion of honeydew. (Photo by Scott Bauer)
(Spanish version PDF file, 1.42 MB)
By Steven E. Naranjo1, Luis A. Cañas2 and Peter C. Ellsworth3
| 1USDA-ARS,
Western Cotton Research Laboratory 4135 East Broadway Road, Phoenix, AZ 85040, USA |
2Dept. of
Entomology Ohio State University Ohio Agricultural Research and Development Center 1680 Madison Ave. Wooster, Ohio 44691, USA |
3University
of Arizona Department of Entomology Maricopa Agricultural Center, 37860 W. Smith-Enke Road, Maricopa, AZ 85239, USA |
(An article recently published in Horticultura Internacional, February 2004, pp.14-21)
This article presents the results of research only. Mention of a proprietary product does not constitute endorsement or recommendation for its use by USDA
The sweetpotato whitefly, Bemisia tabaci (Gennadius) (Fig.
1), was described over 100 years ago as a tobacco pest in Greece
and has since become one of the most important pests of world agriculture.
In addition to direct feeding damage by adults and nymphs, the insect
is known to vector over 110 plant viruses, causes debilitating plant
disorders of unknown etiology and, by the excretion of honeydew, reduces
the quality of harvested products. There exist many challenges to the
development of economically-efficient and environmentally-sound management
systems for B. tabaci. The insect has a reported host-range
of well over 500 plant species, a high reproductive rate, the ability
to readily disperse among hosts and breed year-round, and a propensity
to develop resistance to many classes of insecticides. Previous distributions
of this insect were limited to regions between the 30th parallels. However,
in the past two decades, B. tabaci has invaded every continent
in the world except Antarctica, and commercial trade has facilitated
the regular occurrence of populations in temperate greenhouse production
systems throughout Europe, Asia, and North America. To complicate matters,
B. tabaci is currently believed to be a species complex with
many different recognized biotypes, each having distinct biological
characteristics such as host-plant affinities and differential abilities
to vector certain plant viruses. In Spain, B. tabaci has been
known to occur since the 1940’s, however it has only been in the
last 10-15 years that this insect has assumed the status of key pest,
particularly in the agricultural production region along the southern
Mediterranean coastal areas of Andalucia and Murcia, and the Canary
Islands.
The transmission of viruses such as yellow tomato leafcurl virus, cucurbit
yellow stunting disorder virus and others to important horticultural
crops has been the main cause of concern during the last decade. The
invasion of the B-biotype of B. tabaci has been repeatedly
associated with dramatically increased agricultural and horticultural
problems throughout the world and its invasion into Spain is no exception.
In addition, the discovery of the Q-biotype of B. tabaci in
Spain in the mid to late 1990’s has exacerbated the pest problem
because of the even higher propensity of this biotype to resist insecticides,
most notably the widely-used neonicotinoid compounds such as imidacloprid.
Since the early 1990’s we have been conducting research to understand
the biology and ecology of B. tabaci and to develop integrated
management systems for this pest in Arizona, USA. These efforts have
largely focused on cotton, the primary host of whiteflies during the
summer; however, we learned early on that successful management in cotton
is closely tied to an understanding of pest dynamics in other crop and
non-crop hosts within cotton production areas.
The sweetpotato whitefly has no quiescent or diapause stage and immature
forms are immobile. Therefore, B. tabaci adults reproduce continually
throughout the year by moving sequentially among various crop and non-crop
host plants (Fig. 2).
During the winter in affected areas of Arizona, B. tabaci is
found on vegetables such as broccoli, cauliflower and lettuce, and various
winter weeds. Late winter and early spring hosts include cantaloupes,
vegetables, and weeds. Cotton is the most abundant and favored cultivated
host during the summer, and fall cantaloupes and vegetables complete
the yearly cycle. Perennial crops such as alfalfa and citrus, and ornamental
hosts such as Lantana, and Hibiscus host whitefly
year-round.

Figure 2. The typical seasonal cycle of
Bemisia tabaci in a multi-crop system in Arizona. Populations of the
insect utilize a wide range of hosts over the course of a single year.
Populations persist at low levels during winter months and reach outbreak
levels during the summer.
The suitability of various host plants for reproduction and survival
of B. tabaci varies considerably. For, example, cantaloupes
are a highly favored and nutritious host, whereas alfalfa is a somewhat
marginal host plant. Populations of this pest are at their lowest and
most vulnerable levels during winter months and reach outbreak levels
during summer months. Similar seasonal cycles can be found in all subtropical
and tropical areas where the insect persists year-round.
Because B. tabaci is a multiple-crop pest, development of sustainable,
ecologically-based management strategies will depend on a mechanistic
understanding of the factors governing pest population development in
the mosaic of host crops and wild hosts available throughout the year.
This seasonal cycle is highly complex and is governed by a broad array
of spatially and temporally varying biotic and abiotic factors. The
insect must disperse amongst and adapt to a patchwork of potential hosts.
These potential host habitats vary not only spatially but temporally,
because many consist of annual plant species or crops that grow during
brief periods of the year. Once a new host is located the insect must
successfully develop and reproduce on that host before the host or host
plant parts perish. Each host species presents different challenges.
They may differ in seasonality (annual vs. perennial), abundance, and
suitability. Each host species may also react differentially to environmental
factors (e.g. frost tolerance), and support a different complex of natural
enemies.
A large number of mortality factors affect the survival of B. tabaci.
These forces may be naturally-occurring, as in the case of natural enemies,
weather, or host-plant effects, or man-made as exemplified by insecticides
or cultural manipulations within managed systems. An understanding of
the timing, spatial distribution and magnitude of these mortality factors
is central to the study of population dynamics and is also key to predicting
pest outbreaks and developing better pest management systems that take
advantage of existing mortality forces.
Life tables are a convenient and robust method for describing mortality
in a population and for quantifying probabilities of death from various
causes. In the late 1990’s we used a life table approach to study
the dynamics of B. tabaci populations in Arizona cotton during
the summer and to understand the complementary role of selective insecticides
and natural enemies in pest population suppression.

Figure 3. Mortality of Bemisia tabaci
was studied in the field by marking the location of individual eggs
and nymphs on host leaves and frequently observing the fate of each
insect with a magnifying lens until it died or became an adult. (Photos
by Steve Naranjo and Luis Canas)
In 2000 we expanded our focus to include study of the population dynamics
of B. tabaci as it moves from one host to another throughout
the entire year. Our goals were to compare and contrast the mortality
factors affecting populations of B. tabaci at different times
of the year as a means of providing a mechanistic understanding of the
pest’s population dynamics over a wide area.
With this and other knowledge we hoped to better exploit weak links
in the insect’s seasonal cycle leading to improved and more sustainable
pest control. Study sites were established in three geographically and
climatically distinct areas in Arizona where agricultural production
occurs. In each area we established whitefly “ecosystems”
consisting of a sequence of six representative hosts including winter
broccoli, spring and fall cantaloupes, summer cotton, perennial alfalfa,
various annual weeds, and the perennial ornamental, Lantana.
None of the cultivated crops were treated with insecticides. Within
each of these host plants we measured the population levels of whiteflies
and their associated arthropod natural enemies. We also used life tables
to identify and quantify the mortality factors affecting immature stages
(eggs and nymphs) of B. tabaci on each host plant. This article
will focus on this latter aspect of the research.
To study mortality, we took advantage of the immobile nature of most
of the insect’s immature stages. Eggs are laid individually, generally
on the undersurface of the leaf, where they remain until the hatching
of the crawler, the only mobile immature stage. The crawler which is
actually a young 1st instar nymph, generally moves a very short distance
(several centimeters) before settling 3-6 hours later. Once settled,
this 1st instar nymph and all subsequent nymphal stages remain at exactly
the same location on the leaf.
We marked the location of individual eggs and settled 1st instar nymphs
on leaves and then observed them every few days with a magnifying lens
to determine developmental stage or cause of death if the insect died
(Fig. 3). These studies were conducted multiple
times on each host plant throughout 3 years of study.
 |
 |
Figure 4. A) An adult big-eyed bug,
Geocoris punctipes, and B) a larval green lacewing, Chrysoperla sp.,
preying on Bemisia tabaci. These species are a few of the many generalist
predators found in agricultural systems in Arizona and elsewhere. (Photos
by Jack Dykinga)
As noted, many factors can cause mortality of immature whiteflies, including
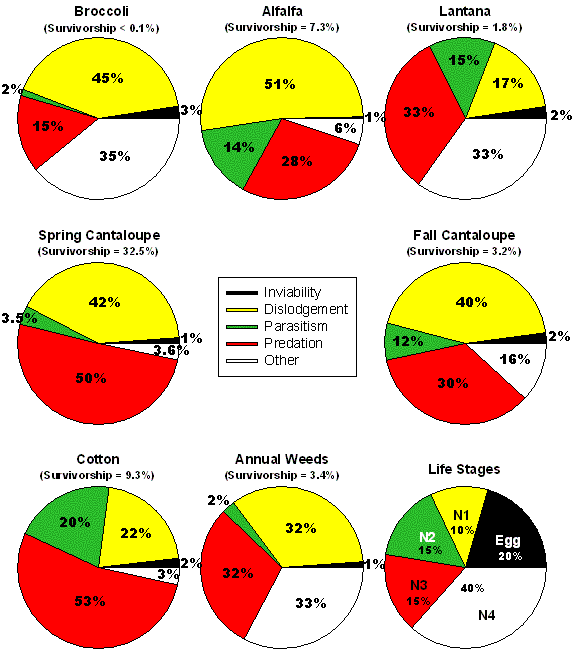
natural enemies such as predators (Fig. 4 and 5),
parasitoids and pathogens, weather factors such as rain and wind, and
other host plant and natural physiological factors. We developed life
tables to quantitatively estimate the effect of each factor and to identify
the developmental stage they affected. Summary results from a number
of whitefly generations observed at one of our study sites in central
Arizona are presented in Fig. 6. These pie charts
show the relative contribution of each factor to total mortality of
eggs and nymphs; the overall immature survival rate is shown above the
pie chart. The factors causing mortality are the same in all host plants;
however the relative contribution of each individual mortality factor
varies considerably among host plants and the time of the year that
they are grown.
 |
 |
 |
 |
 |
 |
 |
 |
 |
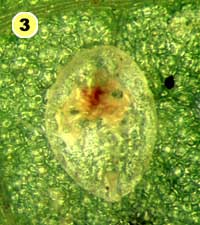
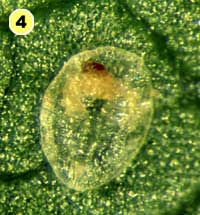
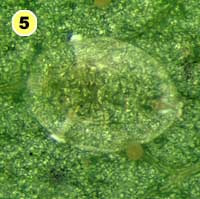
Figure 5. Examples of mortality factors affecting nymphs of Bemisia tabaci. 1) Paecilomyces fumosoroseus is one of several fungi attacking whiteflies; we did not observe mortality from fungi in our study (photograph by Stephen Wraight). 2) Evidence of predation by a predator with chewing mouthparts. 3) Predation by a larva of the green lacewing, Chrysoperla carnea; note the paired puncture marks where the predator inserted its sickle-like mouthparts. 4) Predation by the minute pirate bug, Orius tristicolor. These sucking predators often leave a small amount of the whitefly body contents behind. 5) Predation by the big-eyed bug, Geocoris punctipes; the body contents are completely removed leaving behind only a transparent shell. 6) Parasitism by an early larval stage of Eretmocerus sp. or Encarsia sp. The parasitoid larva cannot be seen, but the paired yellow structures (mycetomes) normally found in the center of the whitefly body are displaced towards the outside of the body. 7) Pupal stage of Encarsia sp.; note the orange colored pellets toward the outside of the whitefly body which are waste products from the parasitoid. 8) Pupal stage of Eretmocerus sp.; note the absence of any waste pellets. The presence or absence of the waste pellets can be used to separate the two types of parasitoids once they have reached the pupal stage. 9) A parasitoid that has been subsequently eaten by the predator Geocoris punctipes; this sometimes makes it difficult to determine the actual cause of death of the whitefly. (All photographs by Steven Naranjo, except photo 1 which is by Stephen Wraight)
In broccoli, a winter crop, most of the mortality is caused by physical
dislodgement of insects from the plant surface and the effects of low
temperature causing desiccation (other category). Dislodgement is probably
related to the action of predators with chewing mouthparts, but may
also be related to weather factors such as wind and rain. Some mortality
of whiteflies in broccoli is caused by predators with sucking mouthparts,
while only a tiny amount of mortality can be attributed to parasitism
and the failure of eggs to hatch. There is essentially no survival of
immature B. tabaci in broccoli. Alfalfa is present year-round,
however, whiteflies are found in this crop mainly in the fall. Again,
dislodgement of insects from the plant surface by chewing predators
and weather, and the activity of sucking predators are the main causes
of mortality. Some mortality is caused by parasitism while mortality
from other causes and egg inviability is rare. Average survival from
egg to adult is around 7% on alfalfa.
Figure 6. Summary of mortality factors affecting
Bemisia tabaci on different host plants in the field throughout the
year. Each pie chart shows the relative contribution of each factor
to the total mortality of eggs and nymphs; the overall immature survival
rate is shown above the pie chart. The chart on the bottom right shows
the distribution of mortality among eggs and the four nymphal stages
of the whitefly averaged over all host plants.
Whiteflies on another perennial host, ornamental Lantana, are
subjected to high levels of predation and other factors, primarily desiccation
during late fall and winter months. Parasitism and dislodgement of immature
whiteflies were moderately high in Lantana. In fact, Lantana
is relatively frost sensitive and cold temperatures caused plants to
defoliate during December and January leading to high levels of death
through dislodgement during this time of year. On average, less than
2% of eggs become adults on Lantana. Immature whiteflies on
cantaloupes grown during the spring are mainly killed by dislodgement
and predation with very little mortality from parasitism, egg inviability
or other causes. However, on average, nearly 33% of all eggs laid on
spring cantaloupe become adults. The relative contribution of various
mortality factors is similar in cantaloupes grown during the late summer
and fall; however, in this case overall immature survival is just over
3%.
Cotton is the primary cultivated host of B. tabaci during the
summer in Arizona and by far the largest source of mortality on this
host is predation. Parasitism and dislodgement also contribute moderate
levels of mortality. We suspect that much of the dislodgement mortality
is caused by severe thunderstorms that are typical of Arizona during
mid to late summer months. These storms are often characterized by high
winds, blowing dust, and sometimes heavy rainfall. On average, just
over 9% of all eggs become adults in cotton.
Finally, a sequence of annual weeds act as host plants for whiteflies
throughout the year. The particular weeds we examined include ground
cherry (Physalis wrightii A. Gray), cheeseweed (Malva parviflora
L.) and sowthistle (Sonchus asper {L.} Hill). Dislodgement,
predation and other sources of mortality contributed relatively equal
amounts of mortality, with rates of parasitism and egg inviability being
very low. The average immature whitefly survival rate on weeds is roughly
3%. If we examine mortality on a stage by stage basis over all host
plants, we find that much of the mortality occurs during the 4th and
final nymphal stage, with relatively equal rates of mortality in eggs
and the other three nymphal instars.
Our year-round studies with this polyphagous pest have taught us to
appreciate the importance of an areawide perspective for pest management.
Populations in one crop or host are inexorably tied to those surrounding
it in space and in time. Populations of B. tabaci are extremely
low during winter months in Arizona. Despite this fact and the high
levels of natural whitefly mortality that we observed on all host plants,
populations inevitably increase during the spring and reach outbreak
levels by late summer.
This phenomenon emphasizes the high reproductive capacity of this pest
insect, but also highlights the important contribution of crops such
as spring cantaloupes that facilitate the release of B. tabaci’s
biotic potential. It has been estimated that a single B. tabaci
female can lay from under 100 to over 400 eggs in her short lifespan,
depending on host plant and environmental variables such as temperature.
With such high rates of reproduction, immature mortality rates of greater
than 98-99% would be required to maintain stable populations and even
greater rates would be needed for economic pest suppression.
We know from similar life table studies in cotton that insecticides
can provide the additional mortality that is needed to suppress pest
population growth. We also know that the choice of insecticides to use
is crucial.
If we sparingly use more selective materials such as the insect growth
regulators buprofezin and pyriproxyfen, then we achieve season long
control from the combined and complementary activity of natural enemies
and insecticides.
If we use an insecticide with broad toxicity then we rely almost entirely
on repeated applications of these materials for pest suppression because
many natural enemies are killed and can no longer contribute important
levels of mortality.
We have been able to provide only a glimpse of our overall research
effort that is focused on attempting to understand the complex dynamics
of B. tabaci within our multi-crop agricultural system in Arizona.
Through an areawide lens, our results provide insight for improved management
of all affected crops through pest avoidance. For example, better weed
management, elimination or delayed planting of key crops in certain
areas, and enhancing natural enemy populations through conservation
or augmentation may help to prevent population outbreaks in individual
fields and the overall region.
Our results also may improve models for forecasting population growth
and outbreak leading to improved scheduling of control activities. Finally
our results have broad implications for the design of crop distribution
patterns and areawide management approaches.
Issued in furtherance of Cooperative Extension work, acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, James A. Christenson, Director Cooperative Extension, College of Agriculture and Life Sciences, The University of Arizona.
The University of Arizona is an equal opportunity, affirmative action institution. The University does not discriminate on the basis of race, color, religion, sex, national origin, age, disability, veteran status, or sexual orientation in its programs and activities.
Any products, services, or organizations that are
mentioned, shown, or indirectly implied in this web document do not imply
endorsement by The University of Arizona.
Questions concerning this publication can be addressed to:
Steven E. Naranjo snaranjo@wcrl.ars.usda.gov
Luis A. Cañas canas.4@osu.edu
Peter C. Ellsworth peterell@ag.arizona.edu
Cotton Insects | Cotton Insect Pubs | Cotton Insect Data | Stickiness | Advisories | Pesticides | Photos
Home | Cotton | All Insects
document located at: http://cals.arizona.edu/crops/cotton/insects/wf/horticultura0204.html
Copyright © 2001 University of Arizona,
College of Agriculture and Life Sciences
Webmaster: Al Fournier (acis@ag.arizona.edu)