 The Nitrogen Cycle - March 7, 2007 Jeff Schalau, Associate Agent, Agriculture & Natural Resources, Arizona Cooperative Extension, Yavapai County Nitrogen is an essential plant nutrient and is probably the most limiting factor affecting the size of plants in humid ecosystems. In water conserving landscapes, a lack of nitrogen and limited water is a winning combination that toughens plants and makes them more drought resistant. In natural ecosystems, nitrogen is usually in balance with other nutrients and precipitation. The cycling of nitrogen within ecosystems is one of the fundamental relationships that create a sort of balance in nature. All living cells need nitrogen to make nucleic acids, proteins, and other cellular constituents. Plants absorb nitrogen and put them into amino acids and proteins. Other organisms consume the plants. In their stomachs, the nitrogen containing proteins are broken down into amino acids again and recycled to make new proteins and nucleic acids. This can occur a few times if the animals eat other animals. When the plants and animals die, decomposers (fungi, bacteria, protozoa, insects, worms, etc.) break the proteins into plant available nitrogen (nitrate and ammonium) which can be reabsorbed by plants. Around and around it goes. 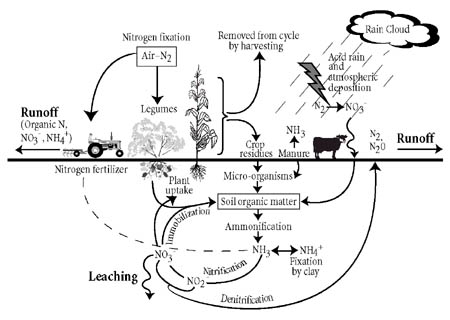 Although the total amount of nitrogen on Earth is fixed, there is an abundant supply in the earth's atmosphere - nearly 79% in the form of nitrogen gas (N2). However, N2 is unavailable for use by most organisms because the two nitrogen atoms are held together by very strong and stable chemical bonds. This makes the N2 molecule effectively inert. In order for nitrogen to be used for plant growth it must be "fixed" in the form of ammonium (NH4) or nitrate (NO3) ions. Microorganisms play an important role in converting the inert atmospheric nitrogen into plant available nitrogen. This process is called nitrogen fixation. Biological nitrogen fixation occurs primarily in agricultural fields (35%), forested lands and wildlands (20%), and the oceans (14%). Non-living factors also convert N2 into plant available nitrogen through lightning (4%), combustion (8%), and industrial fertilizer production (20%). The biological nitrogen fixation process is driven primarily by bacteria which are either free-living or symbiotically associated with plants. The most familiar of these symbiotic associations is between Rhizobium bacteria and plants in the legume family. Some examples of legumes are beans, peas, mesquite trees, catclaw, clover, and alfalfa. Whatever pathway is taken, the nitrogen must be converted from N2 to a plant available form before it can be cycled again. After plant available forms of nitrogen are in the soil, there are three things that can happen: (1) plants may take it up, (2) it may be leached below the root zone by water, or (3) denitrifying bacteria can use it for an energy source and release it back to the atmosphere as N2. The nitrogen released to the atmosphere may start the cycle over again at some point. Any nitrogen leached below the root zone may become a contaminant in groundwater. Gardeners use the nitrogen cycle to their advantage when they compost or through planting cover crops. In a compost pile, the same microorganisms (bacteria, fungi, and protozoa) and invertebrates (worms and insects) present in the soil break down the organic matter into proteins and amino acids. Ultimately, the microorganisms break it down into nitrate and ammonium which can be taken up again by plants. Whether it is a dead plant or manure, soil microorganisms regulate the release of plant available nitrogen from decomposing. Decomposition during the nitrogen cycle is a timed-release process. Decomposition of organic matter by decomposers is regulated by soil temperature – the warmer the soil, the faster the decomposition. Synthetic nitrogen fertilizers are completely available which makes them subject to leaching. These fertilizers are made by exposing N2 in the atmosphere to extreme heat and natural gas which reacts to create ammonia gas. This can be further processed to create ammonium, nitrate, and urea. These are the primary forms of synthetic nitrogen fertilizers that are blended, coated, or otherwise processed to make the products available at nurseries and garden centers. The relationship between nitrogen and living organisms is critical to life as we know it. In wildland settings, nitrogen is often in balance with the ecosystems and local climate. In crop production, nitrogen is managed to increase crop yields. Respect nitrogen and manage it wisely. The University of Arizona Cooperative Extension has publications and information on gardening and pest control. If you have other gardening questions, call the Master Gardener line in the Cottonwood office at 646-9113 ext. 14 or E-mail us at cottonwoodmg@yahoo.com and be sure to include your address and phone number. Find past Backyard Gardener columns or submit column ideas at the Backyard Gardener web site: http://cals.arizona.edu/yavapai/anr/hort/byg/. |