-

- Bead filters: Open style on the left, closed style filters in the back
- Biofiltration
-

-

- Bio Balls Bio-FillTM
-

- A simple degassing column
In the world of aquaculture, filtration and
biofiltration are very distinct and separate entities and they must be
treated as such. Filtration is the removal of solid waste, whereas biofiltration
is the biological process which eliminates toxic nitrogenous wastes.
This page will cover these differences, as well as delving into some of
the numerous types of filtration devices.
- Filtration
One method of removing solid waste from a round fish tank is to use a double drain. It will direct the settled solids to a separate area from the suspended solids. The settled solids can be directed into a small clarifier, much smaller than one which had to be sized to handle the entire flow of recirculating water. The other drain takes the suspended solids along with the nitrogenous waste.
Suspended
solids can be removed by several methods. One is the affinity bead filter
which incorporates the use of small polyethylene beads that have an electrostatic
charge. These beads have an affinity for the negatively charged suspended
solids. As the particles pass these beads, they are "statically" drawn
to them. When the beads are loaded with solids, it is time to backwash
them. (Too often, backwashing is not done frequently enough.) Suspended
solids can also be removed by mechanical means such as bag filters, drum
filters and vegetable filters.
These bacteria use oxygen in a two-step process. The first strain of bacteria, Nitrosomonas, converts the toxic ammonia NH3, to NO2- which is nitrite. Nitrobacter is the second strain of bacteria that converts nitrites NO2- to NO3- which is nitrate. Nitrate is typically tolerated by most cultured species until it reaches very high levels. Controlling nitrate is accomplished by diluting with clean water or by using a denitrification chamber which converts nitrate into nitrogen gas. (This is an anaerobic process that uses a group of chemoheterotrophic bacteria.) A third method that can be used to keep nitrate levels in check is by using plants. You can have a green water system (using algae), a vegetative filter or even use a hydroponic plant filter.
For those of you who are more chemistry minded, here are the nitrification process calculations:
Nitrosomonas calculation - conversion of ammonia to nitrite
55 NH4+ + 76 O2 + 5 CO2
===> C5H7O2N + 52 H2O +54 NO2-
+ H+
Nitrobacter calculation - conversion of nitrite to nitrate
Rotating Biological Contactor: A biofilter converting ammonia to nitrate
- Biofiltration Media
Biofiltration
media is merely a mass of surfaces serving as the attachment basis for
micro-organisms. The spacing between these surfaces is important, both
for the passage of water and to provide sufficient room for bacterial growth.
The biggest cause of biofilter fouling is solid waste, which grows heterotrophic
bacteria. Always try to filter out all solids prior to your biofilter.
Bio Barrels, Bio
Balls, Bio Strata, Bio-FillTM,
scrub pads, and even sand can be used as biofilter media. It is recommended
that you use approximately 300 sq. ft. of surface area per 100 lbs.
of fish in a warm water recirculating system. To give a general idea,
these manufactured media range from 26-370 square feet of surface area
per every cubic foot of media. Another general rule of thumb is having
the volume of the biofilter to be around 15% of the total volume of the
system.
- Stages of Filtration
Regardless
of which type of filtering equipment you decide to use, the important thing
to keep in mind is to always stage your filtration. It is an all to common
mistake to design a system that relies too heavily on a single filtering
device to provide all of the filtering demands that a recirculating system
has. By staging your filtration, you will find your system performing at
or near its peak.
Sedimentation - removal of solid wastes from the water
1. Do whatever is possible to allow fish feces to drop intact into the waste collection area or self cleaning bottom with minimal damage. Minimize the use of pumps, aerators and air diffusers wherever feces is present.
2. Do not pump the waste prior to separation. Design for gravity flow (or siphon) into a sedimentation tank or basin. Splashing and turbulence can attach air bubbles and break apart solids. Feces and food particles smaller than 40 microns may not settle without chemical floculents.
3. Always locate your biofilter after the solids removal system. Solids provide carbon for heterotrophic bacteria which can foul a biofilter and/or reduce its performance.
4. Clean both the settling area and filters at least once a day even if they contain little waste.
5.
If further filtration is required after sedimentation, pump the water
to an affinity bead clarifier or particulate filter.
Sedimentation Basin Design
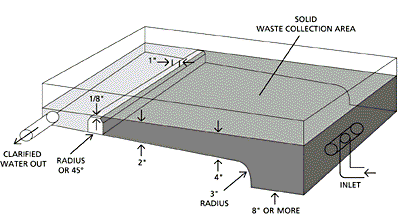
- Here is a good sedimentation basin design: Wide inlet (to reduce
velocity), a surface area of .7 to 1.4 sq. ft. of basin per gpm flow
(for feces with a specific gravity of 1.01 or greater), wide outlet
weir (never a stand pipe), no baffles (which increase velocities)
and a simple waste drain. A depth of just a few inches is enough
for most designs.
- Degassing is a process which is used to remove undesirable gasses,
that are present in greater concentrations in the water than would otherwise
naturally be found. When they are in this state, such gasses are called
supersaturated gasses. A supersaturated gas has a natural tendency, when
exposed to an interface between air and water to escape out of the water
and into the surrounding air. (This is like in fizzy drinks, where while
enclosed in a can and under pressure the gas stays in solution, but when
the can is opened the pressure of the liquid reduces suddenly. The ability
of the drink to hold such an amount of gas is reduced and the gas has a
route to escape through the surface of the drink. The gas forms bubbles
in the glass as it tries to escape.) The main function of a degasser
is to create a large interface between the water and the air. This
is achieved in a variety of ways. One way is by heavy aeration, where
the surface area of the bubbles creates a large interface as they rise
through the water. Aeration also creates a lot of turbulence, bringing
water to the surface where the gas can escape directly to the atmosphere.
Another degassing method is letting the water fall over weirs and cascades,
where it is broken up into droplets, thereby increasing the interface.
Also commonly used are packed columns, which are vessels filled with a
type of media over which the water runs.
Protein Skimmers (Foam Fractionators)
- Protein skimmers are helpful in removing dissolved
organic material from water, and are beneficial for improving water clarity,
aeration and redox potential in marine systems. These units are often
used in conjunction with ozone generators.
Ozone Generators
The use of ozone is increasing in popularity for several reasons:
1. It is highly effective in removing organics, pesticides, color
and nitrites.
2. It reverts back to oxygen quickly. Unlike chlorine, there are
no detrimental residuals (except in saltwater).
3. It is produced on site, with no electricity near the water.
4. It is economical and nonpolluting, when used correctly.
5. It can be used as a sterilizer, before, during and after water
is used for aquaculture.
6. Ozonization improves biological filtration and
particulate filtration.
7. It can remove the biological oxygen demand in the water.
8. It oxidizes long chain molecules, which biofiltration
cannot do.
Ozone is generated by passing air or oxygen through a reaction vessel, where either an electric arc, corona discharge (CD), or an ultraviolet (UV) lamp "excites" the oxygen. In this reaction oxygen molecules separate into atoms of oxygen which then temporarily recombine with each other to form ozone. When ozone oxidizes organics only one atom of oxygen is used, leaving one molecule of oxygen.
Different Types of Ozone Generators
Ultraviolet lights with a specific ozone generating wave length are generally used to produce low levels of ozone. The slower the gas moves through the reaction vessel, the higher the percent of ozone.
The corona discharge (CD) type of uses an electric arc similar to sparks or lightning to produce higher percentages of ozone by weight. A relatively small CD reaction vessel can produce a relatively large volume of ozone. The greater the percentage of ozone, the faster the oxidizing reactions take place.
Ozone can be used in a protein skimmer (foam fractionation device), where it helps the process while the vessel allows capture of the off gas for venting or ozone destruction. Ozone works very well in oxygen saturators for the same reasons.
UV Sterilization
Ultraviolet light can be very effective at eliminating viruses, bacteria, algae and fungi. Since it is the intensity of light that is doing the killing, we must know how much light energy to use and how much is reaching the target. Just as some sunglasses and sunscreens reduce UV intensity, so does discolored water, temperatures, turbidity, dirty quartz sleeves, and even some dissolved salts such as sodium thiosulfate. Old "run down" lamps also affect the intensity. Even lamp temperatures can reduce output when operated in cold water temperatures (110°F is maximum UV output).
To insure sterile water using UV light, you must first start with clear water, have a lamp and flow rate that are sized to deliver the correct amount of irradiation for the target organism (see an exposures list). If a UV light is flow rated for 15,000 mws and you want 30,000 you can either double the amount of lamps or reduce the flow by half.
